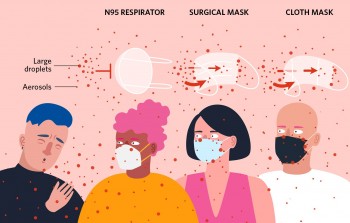
The similarities among surgical masks and surgical N95s are:
- They are tested for fluid resistance, filtration efficiency (particulate filtration efficiency and bacterial filtration efficiency), flammability and bio-compatibility.
- They should not be shared or reused.
Types of respirators - The many types of respirators available include
(1) particulate respirators, which filter out airborne particles;
(2) “gas masks,” which filter out chemicals and gases;
(3) airline respirators, which use compressed air from a remote source; and (4) self-contained breathing apparatus, which include their own air supply.
General N95 Respirator
People with chronic respiratory, cardiac, or other medical conditions that make breathing difficult should check with their health care provider before using an N95 respirator because the N95 respirator can make it more difficult for the wearer to breathe.
Some models have exhalation valves that can make breathing out easier and help reduce heat build-up. Note that N95 respirators with exhalation valves should not be used when sterile conditions are needed.
All FDA-cleared N95 respirators are labeled as "single-use," disposable devices. If your respirator is damaged or soiled, or if breathing becomes difficult, you should remove the respirator, discard it properly, and replace it with a new one. To safely discard your N95 respirator, place it in a plastic bag and put it in the trash. Wash your hands after handling the used respirator.
N95 respirators are not designed for children or people with facial hair. Because a proper fit cannot be achieved on children and people with facial hair, the N95 respirator may not provide full protection.
N95 respirators were originally designed for industrial use in sectors such as mining, construction, and painting.They have also been shown to be effective as protection against engineered nano particles.
Some industrial N95 series respirators have an exhaust valve to improve comfort, making exhalation easier, reducing leakage on exhalation and steaming-up of glasses. Such respirators are not suitable for source control of disease, such as COVID-19, when worn by asymptomatic, but possibly infected users, even if excess N95 respirators are available when healthcare needs are met
N95 Respirator in healthcare
Respirators used in healthcare are traditionally a specific variant called a surgical respirator, which is both approved by NIOSH as a respirator and cleared by the Food and Drug Administration as a medical device similar to a surgical mask. These may also be labeled "Surgical N95", "medical respirators", or "healthcare respirators". As part of the Families First Corona virus Response Act, changes were made to liability and certification laws to allow industrial respirators to be used in healthcare settings, in response to shortages of respirators during the COVID-19 pandemic.
In the United States, the Occupational Safety and Health Administration requires healthcare workers who are expected to perform patient activities with those suspected or confirmed to be infected with COVID-19 to wear respiratory protection, such as an N95 respirator.The CDC recommends the use of respirators with at least N95 certification to protect the wearer from inhalation of infectious particles including Mycobacterium tuberculosis, avian influenza, severe acute respiratory syndrome (SARS), pandemic influenza, and Ebola.
Unlike a respirator, a surgical mask is designed to provide barrier protection against droplets and does not have an air-tight seal and thus does not protect its wearer against airborne particles such as virus material to the same extent.
Email:- sales.livsafe@gmail.com
Phone:- +91 9506020282
Reference